このページの内容は最新ではありません。最新版の英語を参照するには、ここをクリックします。
Battery
汎用バッテリー モデル
ライブラリ:
Simscape /
Electrical /
Specialized Power Systems /
Sources
説明
Battery ブロックは、最も一般的なタイプの充電式バッテリーを表す汎用の動的モデルを実装します。
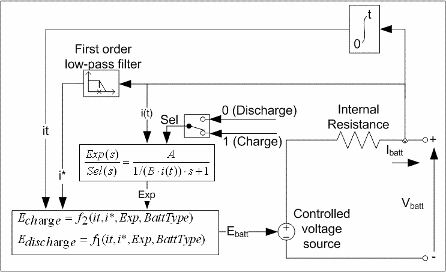
次の図は、ブロックでモデル化される等価回路を示しています。
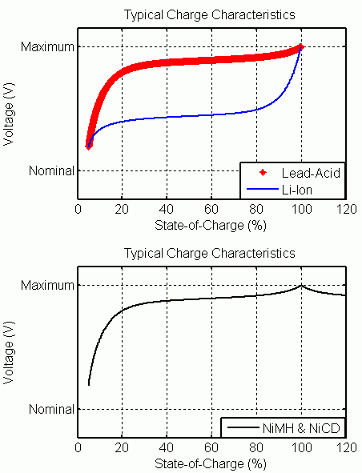
充電特性と放電特性
回路のパラメーターを変更して、特定のバッテリー タイプとその放電特性を表すことができます。一般的な放電曲線は 3 つのセクションで構成されます。
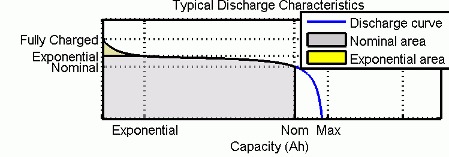
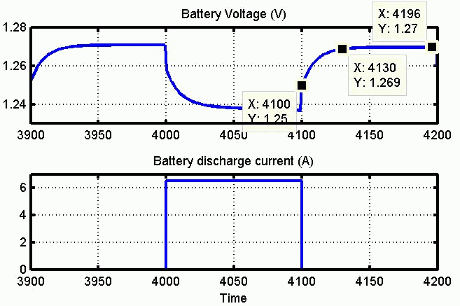
最初のセクションは、バッテリー充電時の指数電圧降下を表します。降下の幅はバッテリー タイプによって異なります。2 番目のセクションは、電圧降下がバッテリーのノミナル電圧を下回るまでにバッテリーから引き出すことができる電荷を表します。最後の 3 番目のセクションは、バッテリーの全放電 (電圧が急速に降下する状態) を表します。
バッテリー電流が負のときは、充電特性に従ってバッテリーが再充電されます。
モデルのパラメーターは放電特性から導出されます。放電と充電の特性は同じであると仮定されます。
Exp(s) 伝達関数は、鉛蓄電池、ニッケルカドミウム (NiCD) 電池、およびニッケル水素 (NiMH) 電池の充電サイクルと放電サイクルにおけるヒステリシス現象を表します。バッテリーの充電状態に関係なく、バッテリーの充電中は指数電圧が上昇します。バッテリーの放電中は指数電圧が即座に降下します。
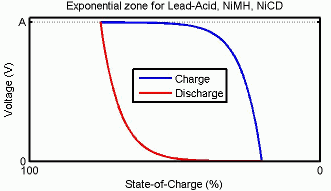
バッテリーの充電状態 (SOC) は、バッテリーの電荷を満充電に対する割合として表した尺度です。放電深度 (DOD) は SOC の補数で、DOD = 100% - SOC となります。
たとえば、SOC に応じて次のようになります。
100% — バッテリーが完全に充電されていて、DOD は 0% です。
75% — バッテリーが 3/4 まで充電されていて、DOD は 25% です。
50% — バッテリーが 1/2 まで充電されていて、DOD は 50% です。
0% — バッテリーの電荷が 0 で、DOD は 100% です。
モデルの検証
モデルの実験的検証では、充電 (電流が 0 ~ 2 C のとき) と放電 (電流が 0 ~ 5 C のとき) のダイナミクスの最大誤差は 5% (SOC が 10% ~ 100% のとき) となっています。
パラメーター化
次の図は、Panasonic NiMH-HHR650D バッテリーのデータ シートから抽出した詳細なパラメーターを示したものです。
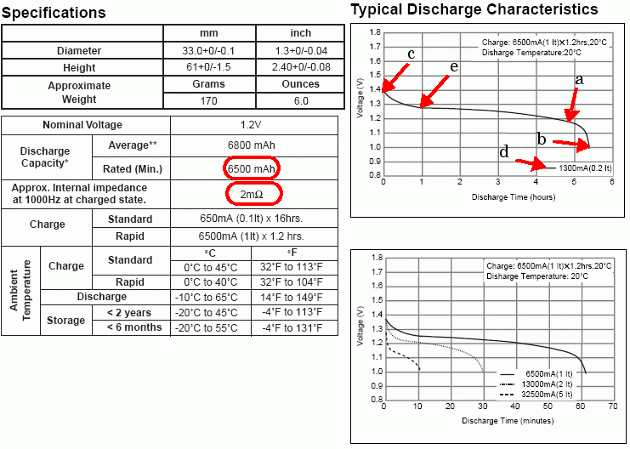
定格容量と内部抵抗は仕様の表から得られます。それ以外の詳細なパラメーターは「Typical Discharge Characteristics」のプロットから導出されます。
パラメーター | 値 |
|---|---|
定格容量 |
|
内部抵抗 |
|
ノミナル電圧 (a) |
|
定格容量 |
|
最大容量 (b) |
|
満充電電圧 (c) |
|
ノミナル放電電流 (d) |
|
ノミナル電圧での容量 (a) |
|
指数電圧 (e) |
|
指数容量 (e) |
|
これらのパラメーターは概算であり、放電曲線から得られる点の精度に左右されます。
これらのパラメーターから得られる放電曲線 (次の図の点線で示した曲線) は、データ シートの曲線に近くなっています。

直接/並列のセルの組み合わせを単一のセルのパラメーターに基づいてモデル化するには、次の表に示すパラメーター変換を使用します。Nb_ser 変数は直列のセルの数に対応し、Nb_par は並列のセルの数に対応します。
| パラメーター | 値 |
|---|---|
ノミナル電圧 | 1.18 * Nb_ser |
定格容量 | 6.5 * Nb_par |
最大容量 | 7 * Nb_par |
満充電電圧 | 1.39 * Nb_ser |
ノミナル放電電流 | 1.3 * Nb_par |
内部抵抗 | 0.002 * Nb_ser/Nb_par |
ノミナル電圧での容量 | 6.25 * Nb_par |
指数領域 | 1.28 * Nb_ser、1.3 * Nb_par |
方程式
鉛蓄電池のタイプには、次の方程式がモデルで使用されます。
放電モデル (i* > 0)
充電モデル (i* < 0)
充電中は it > 0 です。
リチウムイオン電池のタイプには、次の方程式がモデルで使用されます。
放電モデル (i* > 0)
充電モデル (i* < 0)
ニッケルカドミウム電池とニッケル水素電池のタイプには、次の方程式がモデルで使用されます。
放電モデル (i* > 0)
充電モデル (i* < 0)
方程式では、次のようになります。
EBatt は非線形電圧 (V) です。
E0 は定電圧 (V) です。
Exp(s) は指数領域のダイナミクス (V) です。
Sel(s) はバッテリーのモードを表します。バッテリー放電中は Sel(s) =
0、バッテリー充電中は Sel(s) =1です。K は分極定数 (V/Ah) または分極抵抗 (オーム) です。
i* は低周波電流のダイナミクス (A) です。
i はバッテリー電流 (A) です。
it は引き出される容量 (Ah) です。
Q は最大バッテリー容量 (Ah) です。
A は指数電圧 (V) です。
B は指数容量 (Ah−1) です。
制限と仮定
制限
最小無負荷バッテリー電圧は 0 V、最大バッテリー電圧は 2 × E0 に相当します。
バッテリーの最小容量は 0 Ah、最大容量は Qmax です。
仮定
内部抵抗は充電サイクルと放電サイクルにおいて一定であると仮定され、電流の振幅によって変動しません。
モデルのパラメーターは放電特性から導出されます。放電と充電の特性は同じであると仮定されます。
バッテリーの容量は電流の振幅によって変動しません (ポイケルト効果はありません)。
バッテリーの自己放電は表されません。これは、バッテリー端子と並列に大きな抵抗を追加することで表すことができます。
バッテリーにメモリの影響はありません。
端子
出力
保存
パラメーター
参照
[1] Omar N., M. A. Monem, Y. Firouz, J. Salminen, J. Smekens, O. Hegazy, H. Gaulous, G. Mulder, P. Van den Bossche, T. Coosemans, and J. Van Mierlo. “Lithium iron phosphate based battery — Assessment of the aging parameters and development of cycle life model.” Applied Energy, Vol. 113, January 2014, pp. 1575–1585.
[2] Saw, L.H., K. Somasundaram, Y. Ye, and A.A.O. Tay, “Electro-thermal analysis of Lithium Iron Phosphate battery for electric vehicles.” Journal of Power Sources. Vol. 249, pp. 231–238.
[3] Tremblay, O., L.A. Dessaint, "Experimental Validation of a Battery Dynamic Model for EV Applications." World Electric Vehicle Journal. Vol. 3, May 13–16, 2009.
[4] Zhu, C., X. Li, L. Song, and L. Xiang, “Development of a theoretically based thermal model for lithium ion battery pack.” Journal of Power Sources. Vol. 223, pp. 155–164.