カスタム電気化学ドメインを使用した電池
この例では、Simscape™ のサンプル ライブラリ ElectroChem_lib の使用方法を説明します。モデルでは、Fe3+ イオンが還元されて Fe2+ になり、Pb が酸化されて Pb2+ になることで化学エネルギーが放出されます。Pb が酸化されて Pb2+ になるときに 2 つの電子が交換されるため、鉛イオンのモル流量は鉄イオンの半分です。Pb ソースの化学ポテンシャルは固体なので、慣例としてゼロとされます。
電気化学ライブラリはモル流量としてスルー (through) 変数を定義し、化学ポテンシャルとしてアクロス (across) 変数を定義します。この例は次の文献に触発されて作成したものです。F., Allard, B., Lallement, C. & Vachoux, A. & Morel, H., "Modeling and Simulation of Multi-Discipline Systems using Bond Graphs and VHDL-AMS", International Conference on Bond Graph Modeling and Simulation (ICBGM), New Orleans, USA, 23-27 Jan. 2005
モデル
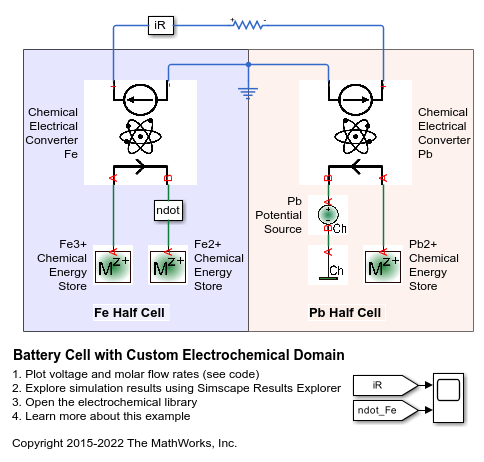
Simscape ログからのシミュレーション結果
